Quality Assurance
Quality Assurance – QA – is the process of verifying whether a product meets required specifications. Many companies use QA testing to gain insight on their current products or to create new systems that will help them maximize their intended goals. Most often, the goal is to prepare a product for State Compliance Testing. QA testing is an advantageous asset in a compliance-regulated industry, which is why we offer the same tests needed at the compliance level a la carte. Clients can pick and choose different tests individually from our menu depending on your testing focus.


Submitting Samples

Sample Requirements

TAT & Results

Useful Info
Our Testing Services

Medium Chain Triglycerides (MCT)
Starting Oct 1, 2024, the CRA requires that all vape cartridges will require testing for medium chain triglycerides (MCTs). Similar to Vitamin E acetate, these fats have some health benefits when consumed orally but are a potential health concern when inhaled.
Infinite CAL is already validated and approved to conduct this testing, which will include the analysis for the four triglycerides to be monitored by the CRA, with an action limit that mirrors that of Vitamin E Acetate.

Cannabinoid Profile
Our cannabinoid potency test provides quantitative information on the active cannabinoids present in a sample.

Residual Solvents
There are dozens of different types of cannabis products, and a number of them require the use of one or more processing solvents during production.

Custom Analysis
Infinite CAL is owned and operated by PhD chemists that have spent their careers developing analytical testing techniques.

Heavy Metals
Heavy metals are associated with serious adverse health effects in humans, ranging from birth defects to cancer.
Caffeine
(any matrix, by HPLC)

Vitamin E Acetate
Vitamin E Acetate Test can determine the presence of this potentially harmful additive in vape products.

Microbial
Microbial Testing is a vital component when it comes to ensuring any major consumer product is free of bacteria and mold that could potentially cause illness.

Stability (Shelf-life)
Using either real-time or accelerated incubation times, shelf-life stability testing allows you to list an expiration date backed up by real data. This should ideally be conducted on all edible products, but is required by the CRA for beverages. Depending on the product type, this can include a variety of safety and quality tests, but at a minimum we suggest testing each product at the beginning and end of its claimed shelf life for microbials and potency.

Terpenes
Terpenes are naturally existing small organic molecules produced by a wide variety of plants, including cannabis.

Kratom
Derived from the leaves of a Southeast Asian tree (Mitragyna speciosa), Kratom has several purported benefits. Our validated assay is capable of quantifying 7 of the most common active ingredients in Kratom products by HPLC, including:
Mitragynine
Mitraphylline
7-hydroxymitragynine
Corynantheidine
Paynantheine
Speciociliatine
Speciogynine

Kava
Used for its sedative properties, the roots of the Kava (Piper methysticum) plant are traditionally consumed in Polynesian countries. The active ingredients in Kava are known as Kavalactones, and Infinite CAL’s validated method is capable of quantifying eight of these compounds by HPLC.
Desmethoxyyangonin
Dihydrokavain
Dihydromethysticin
Kavain
Methysticin
Yangonin
Flavokawain A*
Flavokawain B*

Plant Viroid Testing
Plant pathogens can lead to decreased yield and potency in cannabis plants. Early detection is vital to keep disease from spreading through the rest of your crops. Using molecular detection methods, we are able to test a variety of the most prevalent cannabis plant pathogens in Michigan, including:
Hop Latent Viroid
Lettuce Chlorosis
Cannabis Cryptic Virus
Reach out to our lab if there are other specific pathogens you would like tested. We are capable of testing more specialized pathogens and could meet your grows needs.

Water Activity
The measurement of water activity is a key parameter in the quality control of moisture-sensitive products or materials.

Plant Gender
Just as humans have X and Y chromosomes, cannabis also has a system that determines the plant’s gender.

Pesticides
Because Pesticides are used in the cultivation of cannabis and hemp, they need to be tested for in every product.

Mycotoxins
One of the most prominent dangers to consumers can come in the form of mycotoxins found on or in the cannabis plant and its extracts.

Foreign Materials
The state requires that all cannabis samples be thoroughly inspected using a microscope and visual inspection.

Conversion Testing
Using CBD as a source material, it’s possible to chemically convert the molecule to a variety of isomers of THC. This technique has fueled a rise in the presence of “Hemp-derived” THC that is currently found throughout the U.S., especially in states without a regulated market. This synthetic THC is typically produced without any regulation or testing, and represents a potential threat to both consumer safety as well as a stable (high-THC) Cannabis marketplace.
Infinite CAL has developed a CRA-approved assay to determine if your product originated from THC or CBD, reassuring your customers that your product is derived from the appropriate source material.

Plant Nutrients
(soil, water, or plant material; by ICP-OES)
Plant Tissue
Soil
Fertilizer
Water
Run off
Nutrient
Pythium spp.
Fusarium spp.
Hop Latent Viroid
Heavy Metals
Additional Services
We Offer

CRA Compliance Testing
InfiniteCAL is a fully licensed CRA and ISO/IEC 17025 accredited cannabis testing lab. Regulatory compliance testing is a state-mandated requirement for all cannabis products sold in the State of Michigan.

Colorado Hemp Testing
The Colorado Hemp industry can now count on InfiniteCAL for accurate, reliable, and constant lab results for their compliance testing, as well as any quality assurance testing.
We accept drop-off samples M-F 8 am-7 pm at our lab located in Jackson area and samples sent via FedEx and UPS.
Address:
Infinite Chemical Analysis
4400 Ann Arbor Rd.
Jackson, MI 49202
We can also schedule a pickup for locations across Michigan. To schedule a pickup, please get in touch with your account manager, contact the lab directly or fill out our Request a Sample Pickup Form.
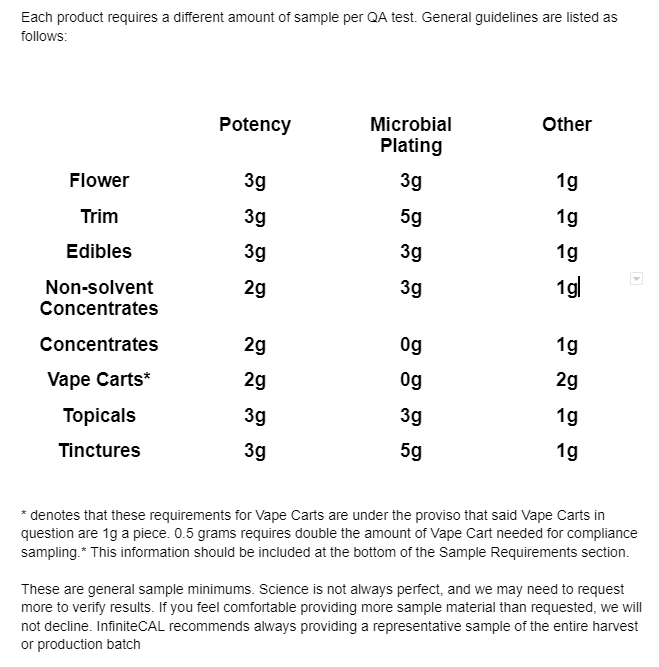
Each product requires a different amount of sample per QA test. General guidelines are listed as follows:


These are general sample minimums. Science is not always perfect, and we may need to request more to verify results. If you feel comfortable providing more sample material than requested, we will not decline. InfiniteCAL recommends always providing a representative sample of the entire harvest or production batch
Results
Results are available wherever you are. Our third-party reporting platform allows 24/7 access to your sample results, unique QR codes, features for sharing COA’s with buyers, and analytical data mining.
Turnaround Time
Our turnaround for QA testing is 2-4 days, which we meet or exceed with a 97% success rate. Expedited options are available for those who need their results by the next business day.

Useful Info
-
QA testing may be requested by anyone 21+.
-
QA tests can be ordered at any time without the official guidelines. Official Licenses are not required for QA testing.
-
Results from QA testing cannot be used to legally sell products to storefronts, but are a good way to know what the status of your products are before submitting them for official tests and can decrease the fail rate for your products.
Cannabinoid Potency Testing
Our potency test provides quantitative information on up to 19 cannabinoids, including phytocannabinoids and synthetic cannabinoids.
Cannabinoids we test for:
Standard Cannabinoid Profile (11 Cannabinoids): Delta-9-Tetrahydrocannabinol, Tetrahydrocannabinol-Acid, Cannabidiol, Cannabidiol-Acid, Cannabinol, Cannabigerol, Cannabigerol-Acid, Delta-8-Tetrahydrocannabinol, Tetrahydrocannabivarin, Cannabichromene, Cannabidivarin
The term “cannabinoid” originally referred to the class of biologically active compounds that occur naturally in Cannabis sativa. The commonalities between these compounds were: a central aromatic ring, two ortho-oxygen atoms on the aromatic ring, and a total of 21 carbons. Now the term takes on a broader definition to include two different subgroups: (1) phytocannabinoids – the compounds that naturally occur in Cannabis sativa – and (2) synthetic cannabinoids – novel compounds that are synthesized in a lab and bear structural similarity to phytocannabinoids.
How we Analyze Cannabinoids
We test for cannabinoids using Ultra High Performance Liquid Chromatography (UHPLC), which separates the cannabinoids and other organic molecules on a column based on interactions with the stationary phase. They then flow through a diode array detector (DAD) which examines parts of the UV-Visible spectrum where the organic compound absorbs light. Depending on the intensity of the absorption, we can analyze the concentration of the cannabinoids present. For more information about cannabinoid potency testing, please refer to pg. 26 of the MRA Regulations.
Moisture Content
Moisture content directly affects potency levels. While the MRA requires that potency is reported by wet weight, ICAL performs this test free of charge on any plant material submitted for potency.
Terpenes (as well as flavonoids) influence the aroma and flavor of these plants, including cannabis. Over 100 terpenes have been detected in cannabis, resulting in countless combinations of smells and flavors.
How We Test For Terpenes
We test for the most common cannabis terpenes using Headspace Gas Chromatography-Mass Spectrometry (HS-GCMS), enabling us to extract and identify each terpene found in a sample. For more information about terpene testing, please refer to pg. ____ of the MRA Regulations.
Terpenes We Currently Test For:
- (-)-Guaiol
- (-)-beta-Pinene
- (-)-Caryophyllene oxide
- (-)-Isopulegol
- alpha-(-)-Bisabolol
- alpha-Humulene
- alpha-Ocimene
- alpha-Pinene
- alpha-Terpinene
- beta-Caryophyllene
- beta-Myrcene
- beta-Ocimene
- Camphene
- cis-Nerolidol
- delta-3-Carene
- d-Limonene
- Eucalyptol
- gamma-Terpinene
- Geraniol
- Linalool
- Pcymene
- Terpino-lene
- trans-Erolidol
Consumer safety is our number one concern. Because growers can use pesticides during the cultivation of cannabis and hemp, they need to be tested for in every product. The MRA requires that all cannabis products sold for consumption be screened for a variety of pesticides.
How We Screen For Pesticides
We use Liquid Chromatography-Mass Spectrometry (LCMS) and Gas Chromatography-Mass Spectrometry (GCMS) to analyze the required 58 pesticide levels within a sample. For more information on pesticide testing, including the action level of each analyte tested, please refer to pg. 28 of the MRA Regulations.
Pesticides We Screen For:
- Abamectin
- Acephate
- Acequinocyl
- Acetamiprid
- Aldicarb
- Azoxystrobin
- Bifenazate
- Bifenthrin
- Boscalid
- Carbaryl
- Carbofuran
- Chlorantraniliprole
- Chlorfenapyr
- Chlorpyrifos
- Clofentezine
- Cyfluthrin
- Cypermethrin
- Daminozide
- DDVP (dichlorvos)
- Diazinon
- Dimethoate
- Ethoprop(hos)
- Etofenprox
- Etoxazole
- Fenoxycarb
- Fenpyroximate
- Fipronil
- Flonicamid
- Fludioxonil
- Hexythiazox
- Imazalil
- Imidacloprid
- Kresoxim-methyl
- Malathion
- Metalaxyl
- Methiocarb
- Methomyl
- Methyl Parathion
- MGK-264
- Myclobutanil
- Naled
- Oxamyl
- Paclobutrazol
- Permethrins
- Phosmet
- Prallethrin
- Propiconazole
- Propoxur
- Pyrethrins
- Pyridaben
- Spinosad
- Spiromesifen
- Spirotetramat
- Spiroxamine
- Tebuconazole
- Thiacloprid
- Thiamethoxam
- Trifloxystrobin
In general, the processing chemicals used to make most cannabis products are harmful when ingested or inhaled. Residual Solvent screening is the only way to ensure that these products are safe for consumption.
How We Test For Residual Solvents
We use Headspace Gas Chromatography-Mass Spectrometry (HS-GC-MS) to quantify the 20 solvents deemed most prevalent and/or harmful by the MRA. For more information on residual solvent testing, including the action level of each analyte, please refer to pg. 30 of the MRA Regulations.
We perform microbial analyses for Inhalable products, Non-Inhalable products and custom plating.
How We Test For Microbial Growth
We use two different methods to test for microbial growth:
The first method is plating, which allows us to identify the total colony-forming units per gram of sample. This well-vetted technique has been utilized for over a century to provide an estimate of the total bacterial or fungal cell concentration within a sample. This method can test for yeast and mold and coliforms. MRA regulation requires Yeast and Mold incubations of at least 72 hours, and has different limits for AU/MED materials as seen on pg. 33 of the regulations.
The second method involves quantitative polymerase chain reactions (qPCR) to determine the presence of harmful microbes within a sample. The process begins by adding a nutrient-rich broth to the sample, making it the perfect living condition for any microbes to grow after an incubation period. For example, salmonella and Shiga toxin-producing E. coli (STEC) require a minimum of 18 hours incubation, while the mold, Aspergillus, needs at least 24 hours of incubation before testing can begin. These extended incubation periods give the microbes (if present) adequate time to grow and replicate to produce enough DNA for our instrument to detect. After the samples are brought out of the incubator, we use validated methods to begin DNA extraction from each sample. After DNA extraction is complete, each sample is set onto a plate that the qPCR instrument will read and use fluorescence to verify the presence of an analyte.
We Offer Custom Microbial Plating For:
- Aerobic Bacteria (APC)
- Enterobacteriaceae
- Coliform
- E. Coli
- Yeast & Mold
We also offer pH testing for samples already in aqueous solution.
Mycotoxins are a secondary metabolite produced by fungi and some molds that readily colonize crops and can cause major health issues for consumers.
How We Test For Mycotoxins
We use Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS) to test for four Aflatoxins and Ochratoxin A. This is not required for regular testing by the MRA, but is available upon request.
Mycotoxins We Test For:
- Aflatoxin B1
- Aflatoxin B2
- Aflatoxin G1
- Aflatoxin G2
- Ochratoxin A
Cannabis plants are more susceptible to heavy metal contamination because they are in a unique class of hyperaccumulating plants that can tolerate uptake of significant levels of heavy metals from soil before their growth cycles are negatively affected. Thus heavy metal testing is vital to ensure consumer safety.
How We Test For Heavy Metals
ICAL uses a state-of-the-art technique called Inductively-Coupled Plasma-Mass Spectrometry (ICP-MS) to quantify these metals. ICP-MS is a highly sensitive technique that detects analytes down to the Part Per Trillion (ppt) range. Typically, a sample is fully digested in a microwave apparatus, diluted, and then injected into the ICP-MS instrument. The sample is then ionized in an inductively coupled plasma flame that reaches temperatures between 6,000K and 10,000K (comparable to temperatures on the Sun!). The resulting ions are separated and quantified by their mass-to-charge ratios (m/z) in a mass spectrometer. For more information on heavy metal testing, including the action level of each analyte tested, please refer to pg. 31 of the MRA Regulations. Current MRA regulations require quantification of arsenic, cadmium, chromium, mercury, and lead in all cannabis and cannabis-derived products. Additionally, nickel is required on all inhaled products and copper on all vaping products.
Heavy Metals We Test For:
- Cadmium
- Lead
- Arsenic
- Mercury
- Copper
- Nickel
- Chromium
If there is too much water in a product, there is a risk of microbial growth and water migrations. This can lead to clumping, changes in consistency, and reduced shelf-life.
How We Test For Water Activity
We use a water activity probe to measure water activity for solid edible and packaged dried flower products as required by the MRA. Water activity is expressed as a decimal to reflect the ratio between the vapor pressure of the consumable itself, when in a completely undisturbed balance with the surrounding air media, and the vapor pressure of distilled water under identical conditions. For more information on water activity testing, including action levels, please refer to pg. 32 of the MRA Regulations.
Foreign Material Inspection
Foreign material can include hair, insects, excreta, or any related adulterant that may be hazardous or cause illness or injury to the consumer. For more information on foreign material inspections, please refer to pg. 31 of the MRA Regulations.
Vitamin E Acetate is a popular diluent & thickener commonly found in illicit vape products. While Vitamin E acetate has been used in dietary supplementation and in the cosmetics industry for years, inhaling the lipid can cause serious lung injury. While there are currently no California regulations regarding testing for the diluent, we highly recommend running this test when sourcing your distillate to protect your consumers and enterprise.
How We Test For Vitamin E Acetate
We’ve established a fully validated method to test for vitamin E acetate using Liquid Chromatography Triple Quadrupole Mass Spectrometry (LC-MS/MS). This test is provided free of charge for all concentrates, although it is only required for Vape Concentrates by the MRA.
However, figuring out genetically the gender of the plant is not as simple as looking for an X and Y.
Luckily, the specific genetic sequence that differs between female and male plants can be identified using quantitative polymerase chain reactions (qPCR) to determine the gender of any plants with 99% confidence.
How We Determine Gender
When a sample is brought in for gender identification, qPCR analysis determines if the plant is female or male. First, a small hole is cut out of the plant’s leaf and added to a lysis solution to destroy the plant cell walls, exposing the DNA. After isolation of the DNA, it is transferred to another plate that contains reagents to amplify and cause the sample to create a fluorescent light that our qPCR instrument then quantifies and determines the gender of the sample based on the amount of fluorescence.










