How to Gain Consumer Trust Through CBD Testing
As inconsistencies in the CBD industry continue to rise, consumers are craving accountability and transparency when selecting which CBD products to put on or in their bodies. For those using CBD to treat certain ailments, properly labeled CBD content and the use of clean, quality materials are especially crucial. Consumers want to be sure they’re receiving the correct dose and, more importantly, that the products they’re using aren’t doing more harm than good.
Our recent CBD case study revealed many CBD manufacturers are mislabeling products, using contaminated materials, and even selling “CBD” products that have no CBD in them at all. Hemp-derived CBD may be federally legal now, but there are currently no regulations for quality assurance or safety testing for CBD products being sold in places other than a licensed cannabis dispensary. It’s up to the manufacturers whether they want to prove the legitimacy of their products to their customers. We’ve come up with 4 key reasons for why CBD brands and manufacturers should go the extra mile when it comes to testing their products.
- Increase trust and transparency through third-party lab testing
In order to increase customer loyalty and trust, many CBD companies and brands are self-regulating their own products by having them tested at an accredited third-party laboratory. Labs such as InfiniteCAL are seeing a surge of new CBD clients submitting samples for cannabinoid, pesticide, mycotoxin, microbial, residual solvent, heavy metal, and terpene analyses to have certificates of analyses (CoAs) readily available to their customers.
The goal behind independent third-party lab testing is to have a neutral, unbiased source to examine a company’s products. This is important in today’s market, as the non-regulated state of hemp-derived CBD more or less allows manufacturers to slap labels on their products and then market and sell them however they please. When a customer asks to see a CoA, manufacturers who have done their due diligence to seek additional testing will have the analytics to stand by when the quality and safety of their products is in question.
- Have CoAs easily accessible to customers
CBD and cannabis companies are making it easier to access CoAs by including QR codes on their packaging that, when scanned with a smartphone, direct customers to the lab’s reporting platform to view the quality and safety test results. InfiniteCAL includes a unique QR code for every compliance and quality assurance sample submitted for this purpose. Customers can verify cannabinoid content and safety results prior to purchase to ensure the product is genuine and safe to consume.
Many online retailers also include pictures or attachments of test results on product pages so customers can view them as they shop.
- Label accordingly
After perfecting your formulation and verifying your results through a third-party lab, it is essential that your product labels reflect that information correctly and coherently.
- One of the most important pieces of information on CBD labels is the total amount of CBD in the entire product, whether it’s a bottle, oral applicator, or jar. Every CBD product label should clearly state the total amount of CBD it contains in milligrams (mg).
- Many CBD product labels will also convey the number and size of CBD servings in each package. This information is generally listed out as “Serving Size” (Ex: ¾ teaspoon) and “Servings Per Container” (Ex: Approx. 32). Manufacturers should also include information on the amount of CBD in milligrams in each serving (Ex: 31 mg).
- Batch or lot numbers should also be listed on all product labels. Batch and lot numbers are a sign of accountability because if there is an issue or recall, regulators or the company can hold that entire batch or lot. Be sure to provide the batch/lot number of a product when having it analyzed by a lab so the label correlates with the information listed on the CoA. When CBD labels have no batch or lot number, it’s impossible to tell when or where that product was made, whether that specific batch or lot had undergone testing, and if the lot the product was purchased from is being recalled.
- Another piece of information to include on CBD labels is the product’s source for CBD. Hemp-derived CBD products can be made using either hemp flower, full-spectrum CBD oil, broad-spectrum CBD oil, or CBD isolate. All are natural and come from the hemp plant, but they differ in the types of compounds they contain. Some low-quality manufacturers may instead list vague terms like “hemp extract” or “hemp oil,” without ever referring to CBD as a tactic to sell products containing very little or no CBD.
- Lastly, a CBD manufacturer’s information should be on a CBD product label, including the company’s basic contact information like an address, phone number, and website. This allows potential customers to research the product and company more thoroughly to make an informed purchasing decision, while increasing transparency between the company and its customer base.
- Know your product
Yes, having those CoAs on hand to show your customers is important, but understanding what those results mean is invaluable. If a CoA shows that a product boasts a wide range of cannabinoids, learn what each one can be used for and communicate that with potential customers. Or, if all safety testing indicates your product was made from quality materials, share how and where they were sourced from with your customers. Knowing your product inside-and-out and demonstrating that to your customer base through trade shows, live demonstrations, website content, press, and social media will dramatically increase consumer trust and transparency. A CoA should not only provide analytical proof that a product is legit, but valuable insight into what makes that product stand out from the competition.
Interested in increasing your CBD company’s transparency through accurate, quality third-party testing? Contact us today to learn how to get started.

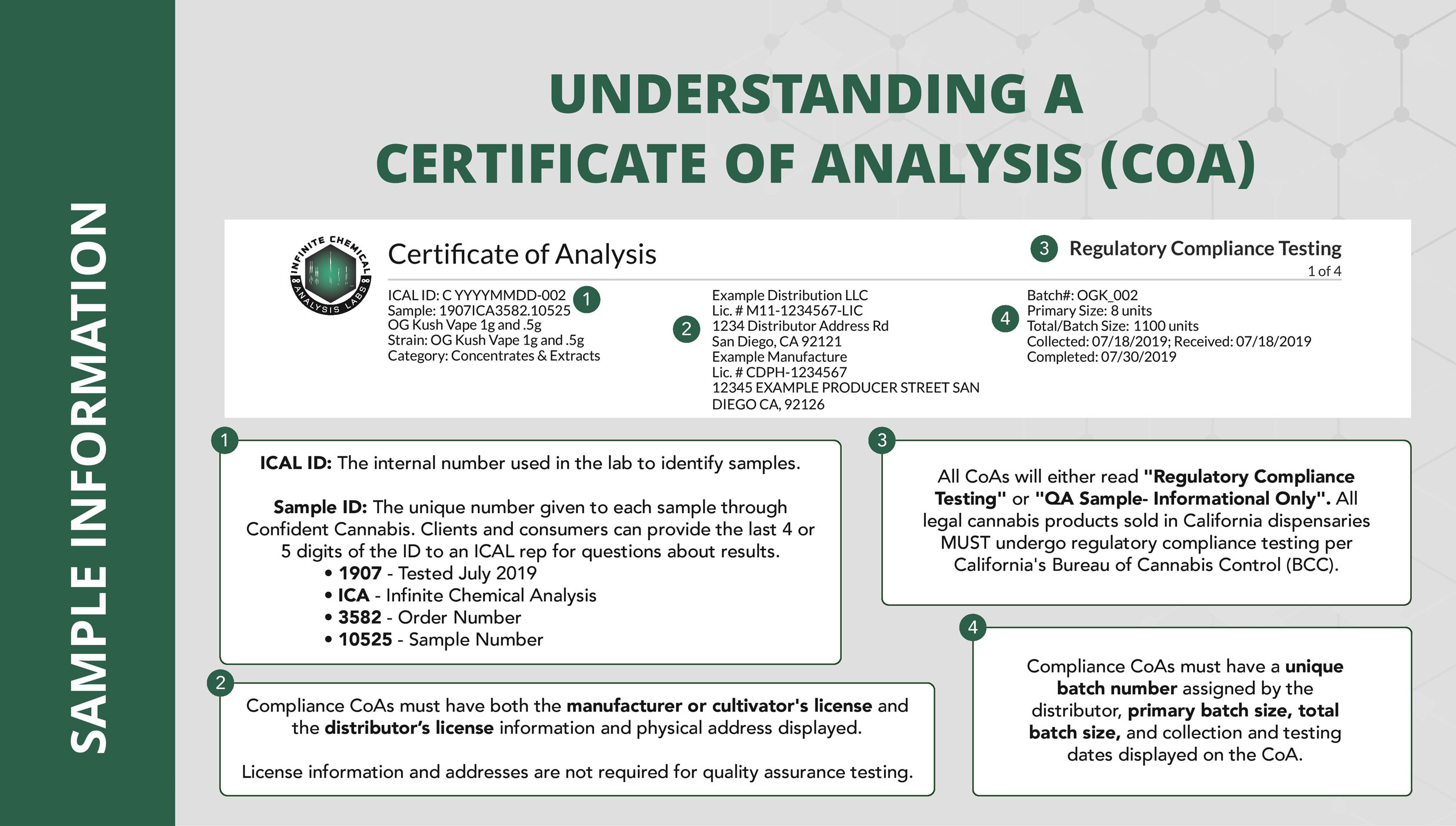
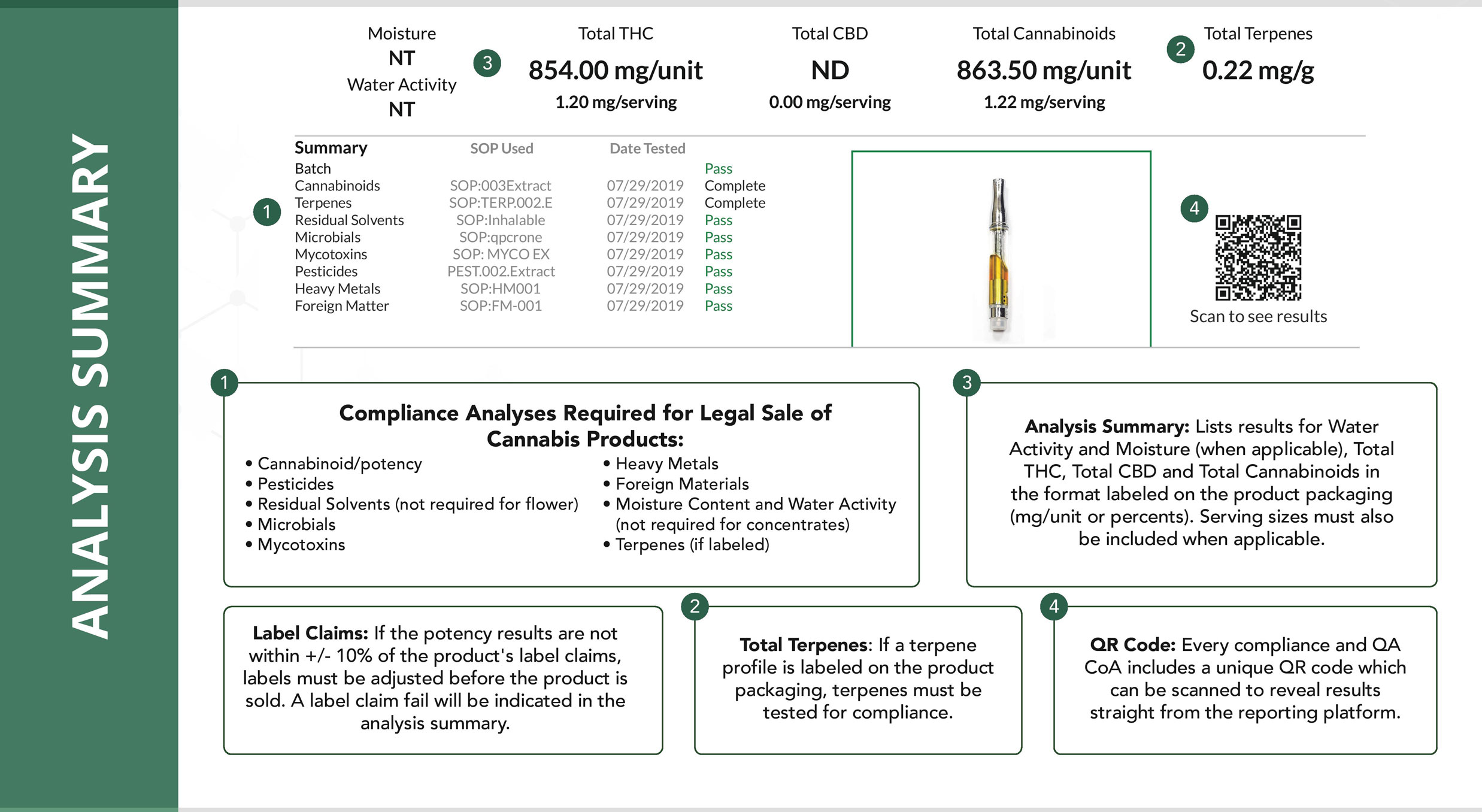
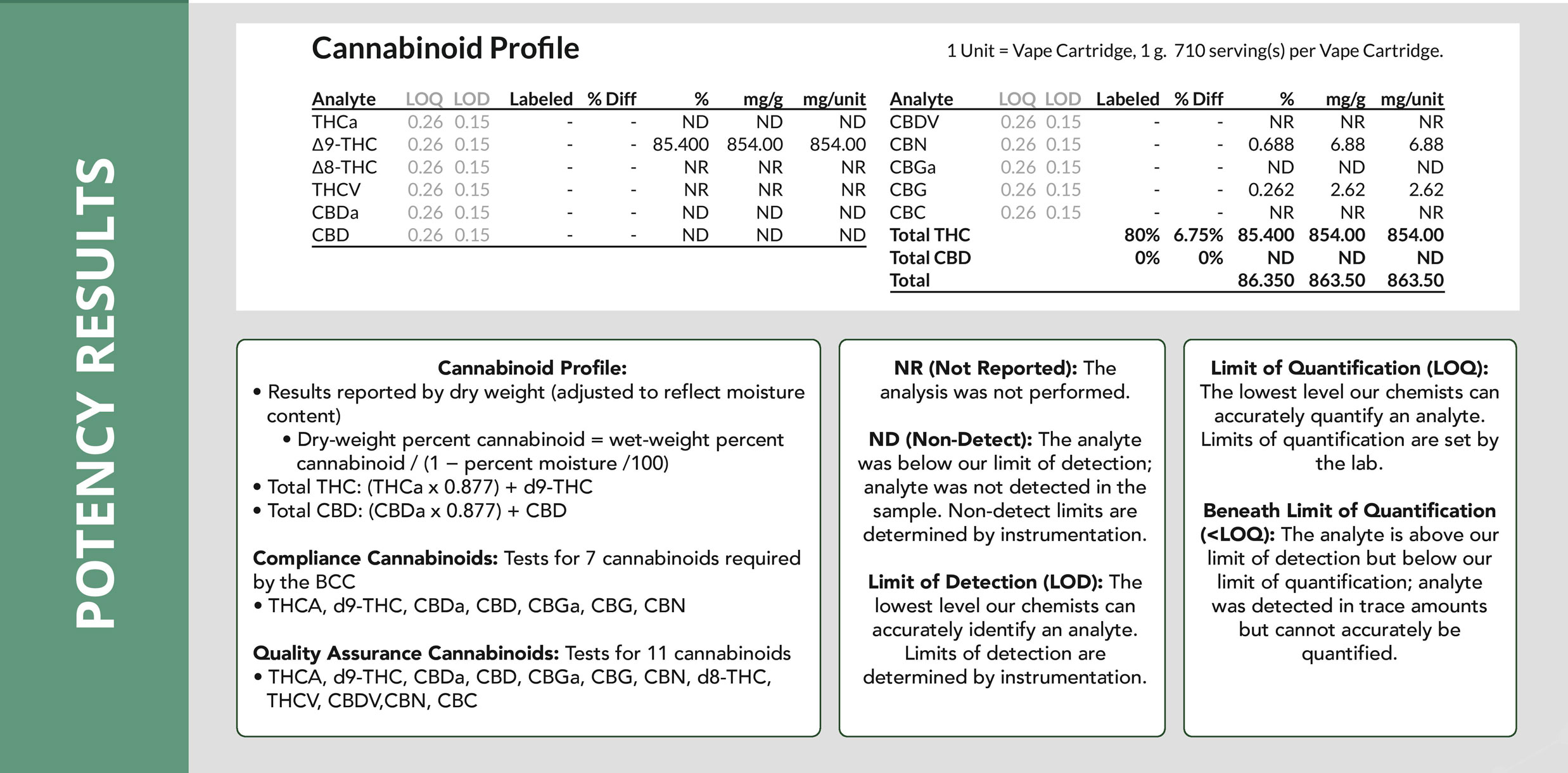
How to Create Test Sample Packages for Regulatory Compliance Testing
The following is a comprehensive guide on how to create test sample packages for regulatory compliance testing on https://www.metrc.com/
- Select Packages from the top navigation

- On the Packages page, select the Active
- Locate and click on the Package sampled by the testing laboratory to highlight it in orange and click on Submit for Testing button above the Packages
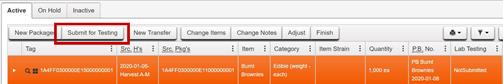
- On the Submit for Testing page, the Package sampled by the testing laboratory is populated in the
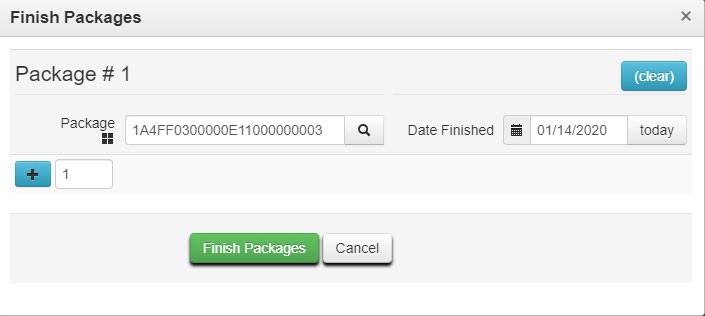
Package #1 field as the source package.
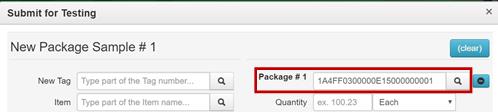
- Enter the representative sample amount obtained by the testing laboratory in the Quantity field for Package #1. Do not enter the entire contents of the source package in the Quantity The remaining quantity in Package #1 after sampling displays below the Quantity field.
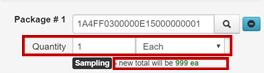
- Assign a Tag to the test sample package. (Provide the whole physical tag to the testing laboratory
– one portion may be affixed to the physical container holding the representative sample, the other portion may be used on the laboratory’s paperwork i.e. chain of custody (COC))
- Assign an Item to the test sample package. If you have not yet created the Item, you must do so before proceeding. Please see the Metrc Manual available in the Support menu for guidance on adding
![]()
- Enter the representative sample amount obtained by the testing laboratory as the Quantity for the test sample package or select the
 button to populate the same amount entered in the Quantity field for source Package #1. Both the test sample package Quantity and the source package Quantity in the action window should be the same amount that was physically taken by the testing
button to populate the same amount entered in the Quantity field for source Package #1. Both the test sample package Quantity and the source package Quantity in the action window should be the same amount that was physically taken by the testing

- An optional Note can be entered, but is not

- Enter the date the test sample package was created in the Package Date field by selecting the
today button.

- After verifying all of the entries, click the Submit for Testing

As a reminder, all test sample packages must also be electronically manifested in Metrc to the testing laboratory.
Example 1 – Creating a Test Sample from a Production Batch Packaged in Individual Units
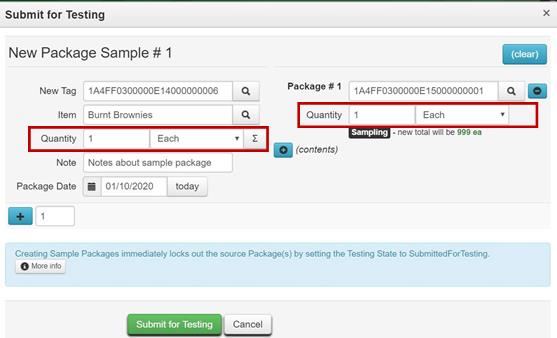
Example 2 – Creating a Test Sample from a Bulk Flower Package
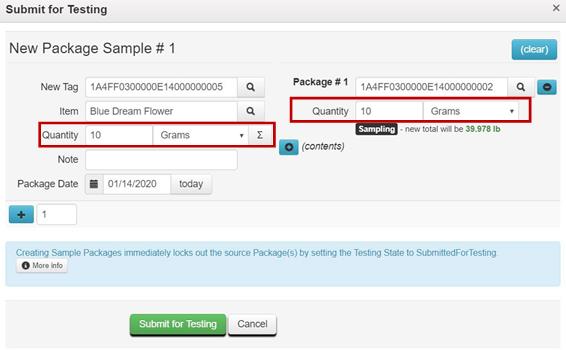
Example 3 – Creating a Test Sample from an “Overweight” Package
When a package exceeds the quantity limit for mandatory regulatory compliance testing i.e. a flower package is greater than 50.0 pounds, the entire contents in the package should be repackaged into two or more new packages that are within quantity limits before creating a test sample.
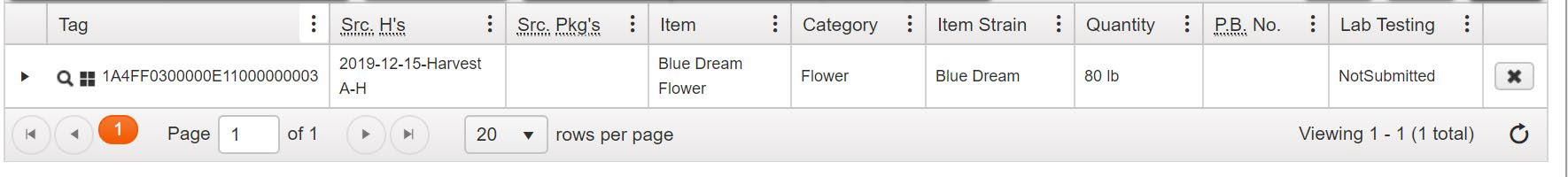
For example, before creating a test sample package from a bulk flower package weighing 80 pounds, the distributor repackages the entire contents of the package into two 40-pound packages.
Package Exceeding Weight Limit:
- Repackage entire contents to distribute the product weight to new packages that are within quantity limits.
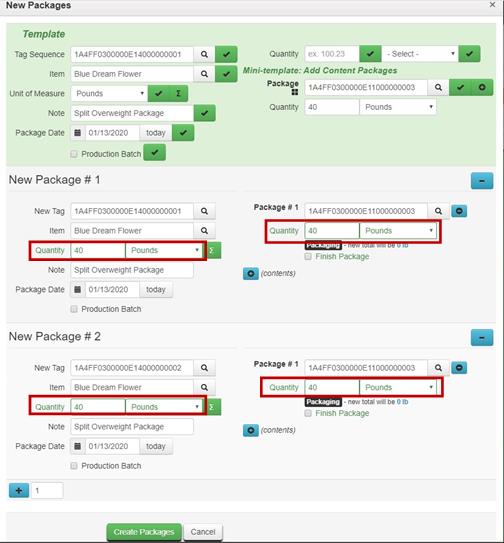
Finish the original source package, which has zero quantity after
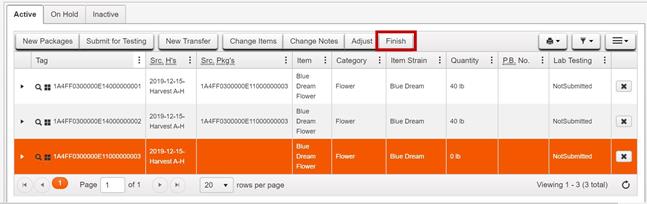
- Create test sample packages from the new packages derived from the original package that are within the quantity limits for testing. See steps and examples above for
Example 4 – Creating a Test Sample Package from Multiple Sizes of the Same Product Containing the Same Production Batch
The testing laboratory may sample multiple manufactured products as one batch, if the batch and recipe is exactly the same and the goods are in their final form including identical packaging/labeling. In the example below, the same production batch oil with the same terpene blend was filled into ½ gram and 1 gram vape cartridges. In this scenario, it is appropriate for the testing laboratory to obtain a representative sample from both packages.
In Metrc, after the representative sample of the mixed sizes is physically obtained, the distributor should electronically pull the number of units from the different sized source packages to create one Metrc test sample package to ensure results are associated to the various sized source packages.
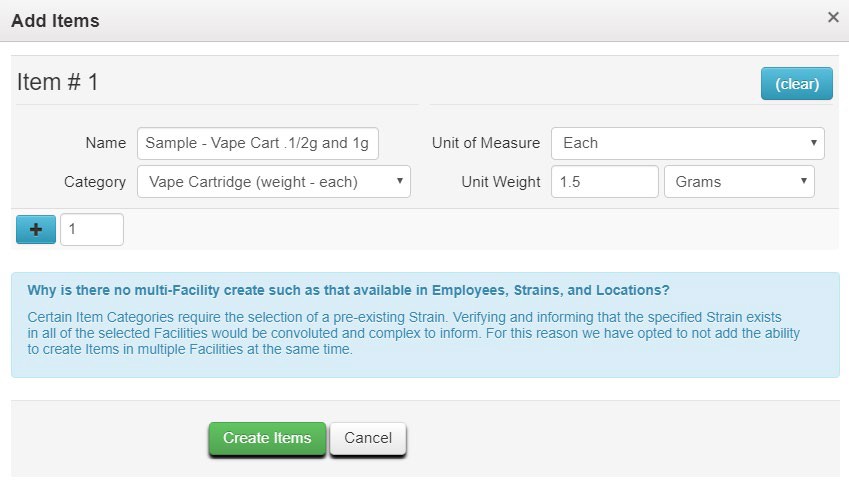
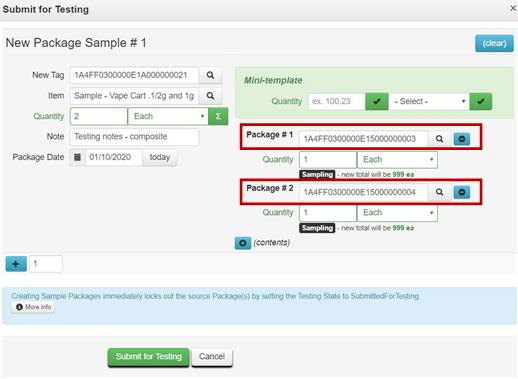
- Before recording the test sample package in Metrc, ensure an Item is available to clearly identify the contents of the combined test sample package. In the vape cartridge example provided above, an Item name such as “Sample – Vape Cart 1/2g and 1g” could be used, and the Unit Weight should reflect the combined weight of the two cartridges (1.5 grams) as shown
- Locate and click on the multiple Packages sampled by the testing laboratory while holding the Ctrl key to highlight both in orange. Click on Submit for Testing button above the Packages
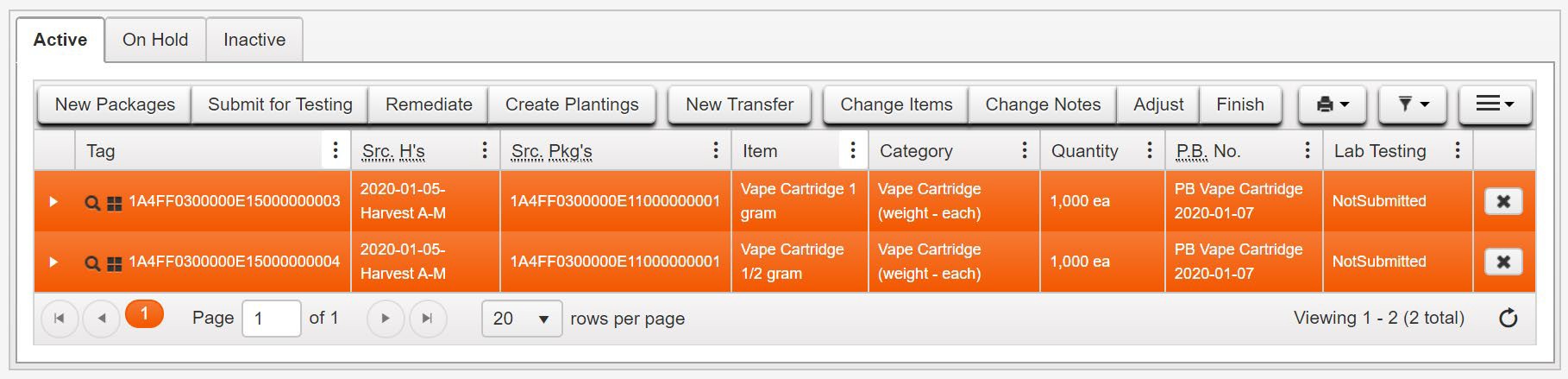
- On the Submit for Testing page, both Packages sampled by the testing laboratory are populated in the Package #1 and Package #2 fields as the source packages for the test sample
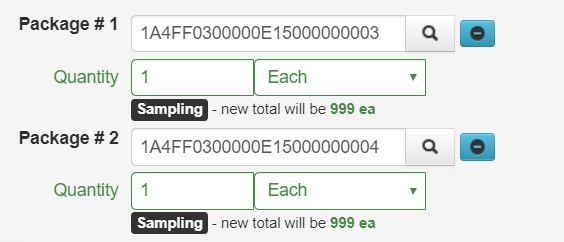
- Follow steps 5 through 11 above to create the test sample
Common Errors and How to Correct
When errors are made during test sample package creation, the ability to move your products, even if the product passed regulatory compliance testing, can be impacted.
Below are some of the most common errors made by licensees and actions you can take to correct those errors.
Example 1 – Creating Multiple Test Sample Packages from the Same Package
Only one test sample package should be created from a package. Having more than one test sample package can cause the Lab Testing State of a package to be set to Testing in Progress after the testing laboratory has recorded the test results on the tested sample, preventing you from moving your product.

Resolution:
If the duplicate test sample package is no longer in your inventory OR the test results have been recorded (Lab Testing State is Testing in Progress), contact Metrc Support for assistance.
If the duplicate test sample package remains in your inventory AND the test results have not yet been recorded on the sample package transferred to the testing laboratory (Lab Testing State is Submitted for Testing), the duplicate test sample package can be discontinued as outlined below. DO NOT adjust the duplicate test sample package to zero and Finish the package as this will guarantee the product will be locked Testing in Progress after all test results have been entered.
- Select the Discontinue
 button on the far right of the row for the duplicate sample
button on the far right of the row for the duplicate sample

- Confirm the discontinuance by selecting the OK button on the pop-up.
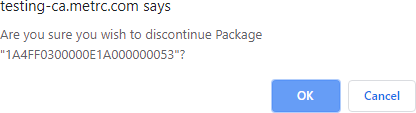
Discontinuing the package automatically returns the contents of the package back to the source package and the package moves to the Inactive tab on the Packages page.
Example 2 – Creating a Child Package from the Source Package Prior to the Testing Laboratory Entering/Uploading Electronic Results in Metrc
You should not create any child packages from a source package until the testing laboratory has entered the numerical test results and the COA is uploaded in Metrc. Once the test results for the source package are recorded and the Lab Testing State is set to TestPassed, a child package can be created and it will inherit the test results of the parent package.
DO NOT create a child package and then pull a test sample from the child package. The test sample should
only be pulled from a source package.
Resolution: If the error is discovered prior to transferring the samples to the testing laboratory, the test sample taken from the child package can be discontinued. See Example #1 for details.
If the samples have already been transferred to the testing laboratory, please contact Metrc Support for assistance.
Example 3 – Pulling from a Test Sample Package to Create a Test Sample Package
Creating a package from a test sample package creates a second sample package. After the testing laboratory has recorded the test results and uploaded the COA on one of the samples, the Lab Testing State of the original source package displays on the Packages grid as Testing in Progress.
Resolution: See the Resolution to Example #1 above.
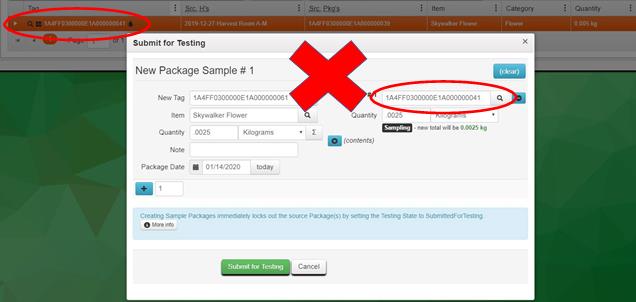
Example 4 – Pulling the Entire Contents of the Source Package into the Test Sample Package
The test sample should be created with a Quantity matching the amount sampled. Verify the new Quantity of the source package when creating the test sample package before clicking the Submit for Testing button. It should not be zero.
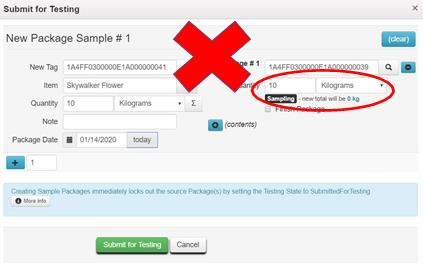
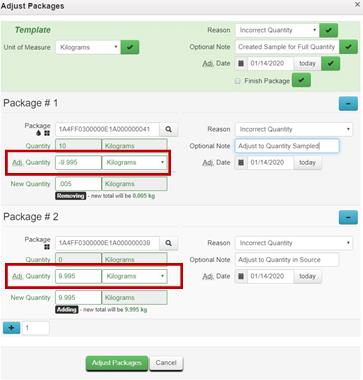
Resolution: While the sample is still in your inventory, discontinue the test sample and remake it correctly. (See Example #1 for steps to discontinue the sample.) If the test sample has been rejected by the testing laboratory because of the discrepancy between the physical sample and the quantity reflected on the manifest, adjust both the test sample package and the source package to the correct quantities.
Please feel free to contact support at [email protected] or 877-566-6506 with any questions.

Consumers: How to Tell if Your Dispensary is Legit
Right?
To the untrained eye, it may be difficult to tell upon appearance. Here are a few ways to ensure your go-to shop is legit:
- Hours of Operation: In California, a dispensary cannot legally sell between 10:00 pm and 6:00 am. If you come across a shop operating late at night, you should steer clear.
- Identification Requirements: Recreational customers must be 21+ in order to purchase cannabis, and cannot buy more than 1 ounce per day. If a dispensary doesn’t ask to see your ID and check you into their system, it’s not a licensed dispensary. Legal delivery services also request some personal information to ensure cannabis products are not being diverted to those under 21.
- Consumption on Store Premises: Just like you wouldn’t crack open a beer in the middle of the grocery store, consuming cannabis on store premises is against the law.
- Tax Fees: If a recreational dispensary is operating legally, they are paying the taxes to do so. Cannabis tax revenue is being used to fund new major social programs, including those for drug abuse prevention, public safety, environmental protection, and economic development. Therefore, there should always be a tax fee displayed on your receipt. Illegal dispensaries are not paying the fees required for operation and are not charging taxes for cannabis purchases, depriving the state of the funding it intended to use for these much-needed programs.
Seem like a lot to remember? The Bureau of Cannabis Control launched a campaign this week that should help clear things up.
The Bureau is now encouraging customers to look for and scan a quick response code (QR code) with their smartphones whenever they go to visit a legal dispensary. Consumers who scan to verify the QR code will be able to confirm that a cannabis retailer is licensed. When scanned, the code will automatically link to the Bureau’s Online License Search and confirms the cannabis retailer’s license status. The system will also display the retailer’s address and license location to ensure that the information is not counterfeit. Retailers have been instructed to place the QR code in storefront windows for easy customer access.
The campaign was prompted but the Bureau’s recent efforts to shut down unlicensed dispensaries. In December, the Bureau and other state agencies worked in coordination with the City of Los Angeles to issue 45 search warrants against illegal operators over the course of three days. The Bureau is hoping a recognizable emblem such as the QR code will help licensed businesses easily distinguish themselves from their illegal counterparts.
An educational video showing consumers how to scan and verify a cannabis retailer’s QR code can be found here. Additionally, retail licensees can learn more about the process for posting their QR code in a fact sheet titled “Information Regarding Posting of QR Codes by Commercial Cannabis Retail Licensees”, which has been posted to the Bureau’s website.
For consumers without a QR code-enabled phone, they can also check a retailer’s license information by visiting the Bureau’s license search tool at CApotcheck.com.

Male vs. Female Cannabis- Why it’s important to know before you grow
Both male and female cannabis plants have their benefits; growing both can result in cross-pollination and thus seeds, resulting in new genetics or seeds for the next crop. However, if your goal is to produce quality buds rich in cannabinoids, it’s crucial to isolate the males from the females to avoid pollination and seed production. Cannabis pollen is extremely potent; studies have shown that pollen can drift across 3 to 7.5 miles, and can reach over 30 miles if high winds are present.
Removing males will allow the female plants to grow abundant, seedless buds (called sensimilla). When female plants are left unfertilized, they use that extra energy meant for reproduction to produce higher levels of cannabinoids like THC or CBD, depending on the strain. The resinous buds consumers purchase at dispensaries are all sensimilla.
How to Visually Determine the Sex of a Cannabis Plant
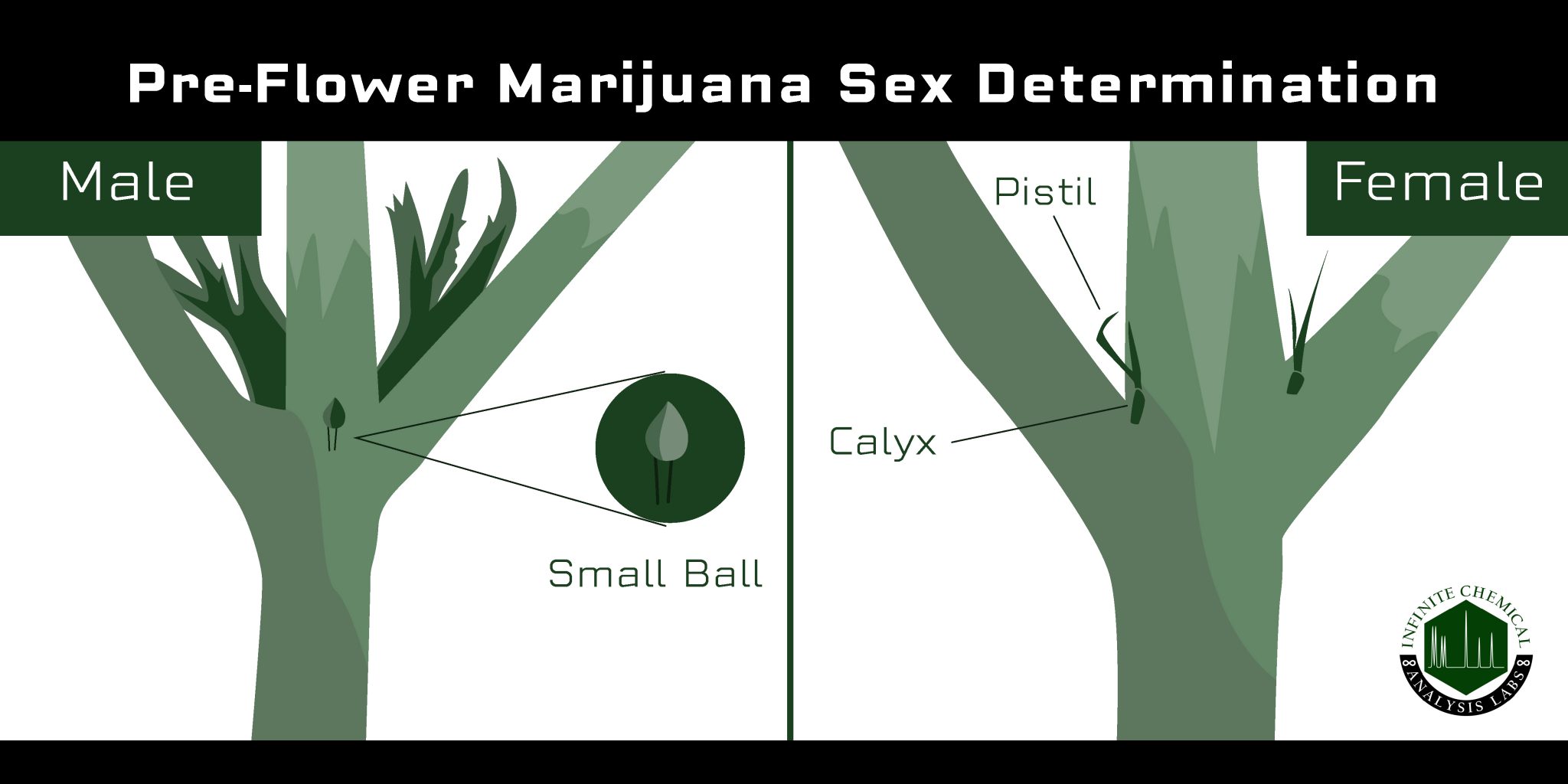
Cultivators can visually determine the gender of their plants about 4-6 weeks into the growth cycle (though this may differ for indoor grows) when the plant is transitioning from its “vegetative” stage into the “flowering” stage. At this time, the plant is no longer focusing its energy on growing bigger and taller and instead spends all its effort growing flowers for pollination and reproduction.
When a cannabis plant is beginning to enter the flowering stage, cultivators should pay careful attention to the area between the nodes of the plant, where the leaves and branches extend from the stalk. Pre-flowers will begin to form in the nodes of the plant, and characteristics of the pre-flower will vary based on gender.
Pre-flowers can initially be difficult to examine with the naked eye, but growers can use a magnifying glass to get a closer look. Female cannabis pre-flowers grow as tiny bracts which will eventually produce hair-like stigma; male plants produce small, round balls as the nodes.
In some cases, a plant may exhibit both male and female pre-flowers. Hermaphrodite cannabis plants can occur when a plant becomes excessively stressed due to things like plant damage, bad weather, disease, nutrient deficiencies, and poor genetics. Hermaphrodites can also produce anthers, often referred to as “bananas” due to their appearance. It’s important to monitor plants that have been exposed to stressors to ensure they don’t begin to develop both male and female genetics. Hermaphrodites are capable of producing pollen and can ruin an entire crop.
How to Determine Gender Before the Pre-Flower stage
Lab genetic testing can determine a plant’s gender as soon as it begins to sprout its second set of true leaves. Knowing sooner can help cultivators save money, increase canopy space, and decrease labor costs associated with transplanting, watering, monitoring, training, and removing unwanted male plants.
Just as humans have X and Y chromosomes, cannabis also has a genetics system that determines the plant’s gender. However, figuring out the gender based on the DNA of a plant prior to the flowering stage is not as simple as looking for an X and Y. Luckily, the specific genetic sequence that differs between female and male plants has long been discovered, so using quantitative polymerase chain reactions (qPCR) allows labs to determine the gender of any plants with 100% confidence.
When a sample is brought in to Infinite Chemical Analysis Labs for gender identification, qPCR analysis is used to determine if the plant is female or male. A small hole is cut out of the leaf of the plant and added to a lysis solution to destroy the plant cell walls, exposing the DNA. After isolation of the DNA, it is transferred to another plate that contains reagents to amplify and cause the sample to create a fluorescent light that our qPCR instrument then quantifies, and determines the gender of the sample based on the amount of fluorescence.
Between sufficient lighting, proper nutrients, a detailed watering schedule, and constant monitoring, identifying the sex of your plants is another tedious yet crucial task that could make all the difference come harvest season.
Gender identification testing is now available at InfiniteCAL to help cannabis and hemp cultivators take the guesswork out of their grow. If you’re interested in learning more about Gender Identification Testing, reach out to our team at [email protected].

Case Study: Illicit Vapes and What You Need to Know
Both bud tenders and Platinum Vape representatives were present to help educate customers about the known risks associated with the illicit cannabis market, specifically the lack of safety testing. All legal cannabis products sold in California must undergo extensive safety testing performed by a licensed laboratory. Vape cartridges specifically are tested for potency, pesticides, residual solvents, microbials, mycotoxins, heavy metals and foreign materials.
California’s cannabis testing regulations are some of the strictest in the nation, and for good reason- since cannabis remains federally illegal, there have been no long term studies done in the United States to examine how different methods of cannabis consumption can affect consumer health. Regulations were set to protect the public from unknown health risks.
However, unregulated products purchased on the illicit market aren’t held to the same standards. Many illegal cannabis manufacturers will cut corners to cut costs, made apparent by the quality of their products compared to those on the legal market. It’s not uncommon to find illicit products riddled with pesticides, heavy metals, microbials and dangerous levels of solvents and diluents used in the manufacturing process. These contaminants may cause seriously compromise consumer health, especially to those whom have weakened immune systems and are using cannabis to medicate.
Twenty-five cartridges collected from customers as part of Platinum Vapes buy-back program were released to Infinite Chemical Analysis Labs to determine just how dangerous these products were. The samples were prepped and analyzed for potency, pesticides, heavy metals, and Vitamin E Acetate, the cutting agent believed to be causing the vaping sickness. Samples passed or failed these analyses based on the Bureau of Cannabis Control’s (BCC) regulations required for all legal cannabis products sold in California.
The cartridges ranged from home-made cannabis concentrates to counterfeits of popular legal brands. While it is unknown how customers acquired these products, an overwhelming 79 percent were deemed unfit for consumption based on the safety analyses conducted. Of the five that passed, two were cut with an unknown diluent, making them questionable at best.
The average legal cannabis concentrate ranges from 60-95 percent THC, depending on the extraction and manufacturing process. That being said, laboratories can conclude a vape cartridge was cut with a diluent based on a simple cannabinoid (potency) analysis. Diluents most commonly used in the illicit market include vegetable glycerin, artificial terpenes, MCT oil, propylene glycol, and tocopherol-acetate (vitamin E acetate). Illicit vape manufacturers use these compounds to cut their cannabis distillate prior to filling their cartridges, so they can double their volume at a fraction of the price. While extensive research still needs to be done to determine whether any of these diluents are safe to inhale, consumers are still being duped into purchasing cartridges claiming 70+ percent THC when they could be containing as little as 0.95 percent, as seen in InfiniteCAL’s results. Half the illicit products InfiniteCAL tested contained less than 50 percent THC, including counterfeits claiming the same 80+ percent of their legal counterparts.
Using liquid chromatography, the samples were also specifically analyzed for Vitamin E Acetate, the popular new diluent used for its similar coloring and viscosity to cannabis extracts. Vitamin E Acetate has been proven by the CDC as the primary cause for the vaping illness outbreak (dubbed EVALI), causing serious respiratory injury and even death to those who have inhaled it. As of December 10, 2019, a total of 2,409 hospitalized EVALI cases have been reported to CDC from all 50 states, the District of Columbia, and two U.S. territories (Puerto Rico and the U.S. Virgin Islands), including 52 deaths.
Almost all of the samples tested had trace levels of Vitamin E Acetate (less than 0.01 percent). However, 5 cartridges contained levels exceeding 20 percent vitamin E acetate, meaning they were purposely cut with the dangerous diluent to increase profit, despite the known consequences for consumer health.
The two safety tests conducted proved even more shocking.
The pesticide analysis conducted by InfiniteCAL screened each sample for 66 pesticides restricted or limited for cannabis cultivation by the BCC using liquid and gas chromatography. Of the 24 samples tested, 62.5% failed for pesticides. In addition, eleven samples contained pesticides with cyano groups that convert into hydrogen cyanide gas when heated, such as myclobutanil, a compound found in the popular Eagle 20 pest control products . For reference, hydrogen cyanide gas chambers have been used as an execution method in the United States.
The samples were then screened for arsenic, cadmium, lead, and mercury using inductively coupled plasma mass spectrometry (ICPMS). These metals are required to be tested for by the BCC because of their known risk of exposure. Chronic inhalation of these metals has been linked to lung, liver, immune, cardiovascular and brain damage, and even cancers.Thirty-seven percent failed for lead, which has an action limit of 0.5 µg/g, though there is no known safe exposure limit according to the US Centers for Disease Controls and Prevention. One sample tested contained an astounding 43.619 µg/g of lead, over 87x the legal limit.
Lead fails are most likely due to the use of cheap hardware purchased from overseas, where the manufacturing standards are not as stringent. Because of a cannabis concentrate’s acidic nature, metals from coils used in these cartridges can be leached out over a period of time, resulting in dangerous levels of heavy metals in the actual product.
Conclusion
Based off the results from Infinite Chemical Analysis Labs, consumers are more likely to purchase a contaminated, dangerous product off the illicit market than not. The cartridges collected by Platinum Vape’s buy-back program came directly from the hands of consumers who may have had every intention of smoking them. Some could have gotten really sick. A few of the carts could have caused a consumer to be hospitalized, or worse.
All cannabis products purchased from anywhere other than a licensed dispensary are unregulated, meaning no quality assurance or safety analysis have been performed per regulations set by the state. Regulations are in place to protect consumers from illnesses such as EVALI. When illicit manufacturers and cultivators decide to disregard these regulations, they’re knowingly putting consumers at risk.
Extensive research still needs to be conducted in order to determine any other health risks associated with vaping. However, manufacturers in the legal cannabis market use only clean, responsibly- sourced cannabis to create their products. Although no regulations for vitamin E acetate have been set in California, analytical laboratories can- and have- deemed a product unfit for consumption if the diluent is detected above 1%, preventing the product from going to market.